Table of Content
As a manufacturer involved in pharmaceuticals, you are likely aware of the criticality of dispensing drugs correctly. Any mistake during this phase may have severe consequences on the health and safety of consumers, not to forget the loss in revenue and credibility for your brand.
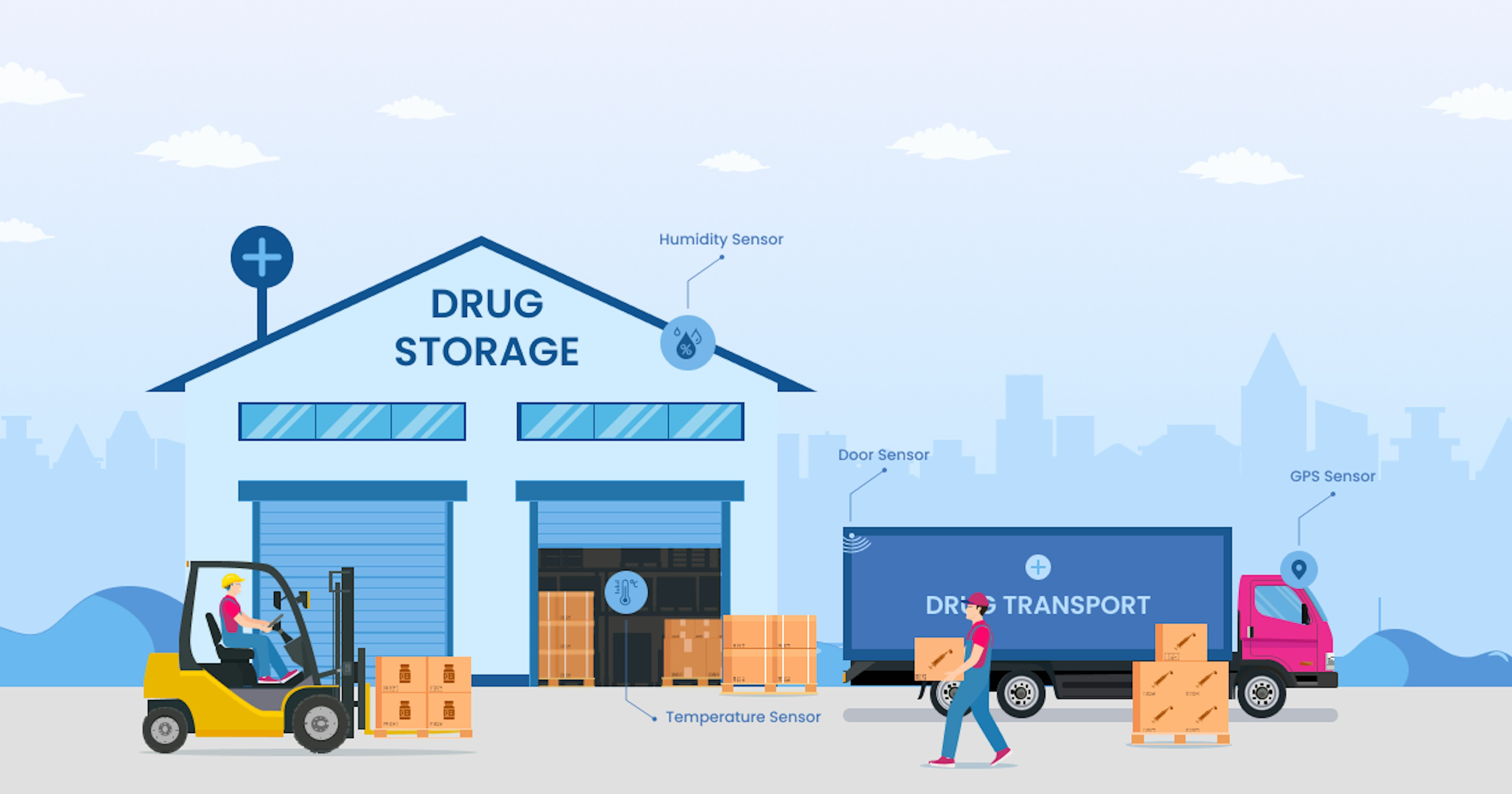
The significance of proper drug storage is also on par with its transportation. Appropriately moving drugs from one location to another is essential for delivering help to consumers. In this regard, it is worth examining how different parameters affect drug storage and transportation and why the disruptive IoT technology is necessary to maintain drug efficacy as intended:
Different parameters to consider in drug storage
Efficient drug storage requires considering factors such as the storage location, compatibility, temperature, air exposure, shelf life, humidity, and light sensitivity of the medication. Some drugs, for example, may require specific storage conditions, such as refrigeration or protection from light, and should be kept in their original airtight packaging until ready to be used. So what kind of trouble do these parameters pose in pharma? Let us find out.
Challenges pertaining to quality control in drug storage and transportation
Condition monitoring here takes center stage because of the extreme sensitivity of drugs to various environmental parameters we studied in the previous section. Therefore, storing and transporting drugs under proper conditions is essential, so they do not become spoiled. However, quality control in drug storage and transportation is marred with challenges, such as:
1. Contamination
Contamination of drugs can occur during various stages of manufacturing, including production, packaging, storage, and transportation. This can decrease drug potency and efficacy and lead to financial losses for pharmaceutical companies and manufacturers.
Therefore, it is vital to follow Good Manufacturing Practices (GMPs), which are a set of guidelines and regulations covering different production phases, including facility design, equipment maintenance, personnel training, quality control, and documentation.
2. Product instability
Any drug that can maintain its robustness, purity, and quality over time is considered "stable." However, this "stability" can be affected by various parameters, such as temperature, humidity, light, and oxygen exposure, resulting in chemical and physical changes in the drug.
3. Quality deterioration
When exposed to high temperatures or light, chemical reactions occur in the medication, resulting in the loss of potency, the formation of harmful by-products, and a decrease in shelf life. Different types of drugs may have varying temperature requirements.
For instance, some medications may require cold storage, while others need to be stored at room temperature. Additionally, some drugs may be sensitive to heat and cold, so maintaining proper storage conditions to ensure their chemical stability is crucial.
4. Drug efficacy variations
Exposure to high/low temperatures and light during the storage and transportation of drugs is also vital. Temperature monitoring using IoT involves implementing data loggers or temperature-sensitive labels that can detect variations and provide real-time data.
Similarly, light-sensitive labels can help determine the level of light exposure, and medications should be stored in opaque or light-resistant containers to minimize exposure to light.
Pharmaceutical companies and manufacturers should ensure that the temperature and light exposure during transit is carefully monitored to prevent damage to the medication. For example, most drugs should be protected from direct sunlight.
They should also be stored in airtight containers to prevent moisture and oxygen from entering. In addition, desiccants, such as silica gel packets, can be added to the containers to absorb excessive moisture.
5. Humidity and oxygen exposure
High humidity levels can cause drugs to absorb moisture, which results in degradation of the medication. When moisture enters the drugs, it can cause chemical reactions that alter its composition, thereby spoiling it for use.
Moisture can also lead to the growth of microorganisms, such as bacteria and fungi, leading to mold growth, which further compromises the quality of the medication.
On the other hand, oxygen exposure can cause some drugs to degenerate, particularly those sensitive to oxidation. Oxygen has the power to react with certain compounds in the medications, leading to a loss of potency and efficacy.
6. Non-compliance with regulations
Regulatory compliance is critical to maintaining the integrity of the drug supply chain. Regulatory agencies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), enforce regulations ensuring that drugs are manufactured, stored, and transported under appropriate conditions to maintain their quality and robustness.
Regulations include guidelines and requirements for the manufacturing process, storage conditions, labeling, packaging, and transportation of drugs. Non-compliance with regulations can result in fines, product recalls, suspension of licenses, or even criminal charges - basically, everything that any pharma company or manufacturer would want to avoid at all costs.
In addition to the financial impact, it can damage the reputation of the stakeholders involved, resulting in a loss of trust amongst consumers and credibility in the market.
IoT solutions that help in the quality control of pharmaceutical storage and transportation
IoT technology is gaining significant traction in the pharmaceutical industry - for a good reason. It has the potential to revolutionize various processes by improving efficiency, quality, and safety. For starters, IoT sensors can ascertain environmental conditions and ensure that they are within the required parameters.
They can track the movement of drugs, from manufacturing to distribution to retail to ensure the products are handled properly, and their quality is maintained throughout the supply chain. Finally, IoT can be used to improve drug safety by ensuring that medications are stored and transported under the proper conditions.
It enables pharma companies to study different parameters in real-time, detect fluctuations in the drug composition, and take corrective action to maintain the stability and effectiveness of the drugs. Let us look at the top IoT solutions that we should know about in this regard:
1. Wireless temperature sensors
These sensors can be placed in different storage facility areas to monitor the temperature and humidity levels. They are connected to a central hub, which collects data from all the sensors and sends it to a cloud-based server for analysis.
This enables pharmaceutical companies and manufacturers to monitor the temperature and humidity levels remotely and receive alerts in case of any deviations from the optimal temperature and humidity ranges.
Smart thermometers specifically can monitor the temperature in drug storage and transportation. They are typically connected to mobile devices, allowing the tracking of temperature levels in real time from smartphones or tablets. Smart thermometers are portable.
2. Alarms and notifications
IoT solutions equipped with alarms and notifications can detect temperature deviations and send alerts in the form of an email, a text message, or a mobile app pop-up to the storage facility personnel in real-time.
They can then immediately address the deviation and ensure drug stability using IoT without compromising patient safety. For example, if the humidity level in the storage facility rises above the recommended range, an alarm can be triggered to inform the concerned personnel.
3. Automated temperature control
This functionality commonly uses a system of sensors, controllers, and actuators to maintain the temperature within the desired range. The system is programmed to adjust the temperature in response to changes in the environment, such as fluctuations in temperature or humidity levels.
IoT temperature control can help maintain the parameter within a narrow range, reducing the risk of fluctuations that can severely impact the quality of a drug. The system is energy-efficient and requires less manual intervention, which means the pharma companies do not have to hire a lot of people for the job, thereby saving costs.
4. Controlled Transportation Unit (CTU)
A CTU is a specially designed container transporting temperature-sensitive goods over long distances. It has a control system that enables remote monitoring and adjustment of various parameters such as temperature, humidity, and air quality.
It uses sensors to measure these parameters. A CTU sends the data to a central control unit for analysis, which then adjusts the container's environment to maintain the required parameters. This enables the transportation of drugs that require a specific environmental condition to remain intact during transit.
A CTU also ensures that products are delivered in the same condition they were in when they left the point of origin, reducing waste and ensuring customer satisfaction.
6. Predictive maintenance
This technique uses ML algorithms to predict and control issues related to temperature and humidity in storage or transportation units to address the potential problems before they result in unwanted deviations and drug spoilage.
Predictive maintenance involves collecting and analyzing data from various sources, such as temperature and humidity sensors installed in the storage or transportation units.
Once potential issues are identified, proactive measures can be taken to address them before they cause any damage to the drugs being transported or stored.
For example, if the predictive maintenance system identifies that the temperature in a storage unit will rise above the acceptable range, the system can automatically activate the cooling system to lower the temperature before it becomes a problem or inform the concerned personnel about the potential deviation so that they can fix it before it becomes a problem.
7. Humidity monitoring
Humidity sensors can be integrated with IoT solutions to monitor the moisture levels in storage and transportation containers. They can detect deviations from the recommended humidity range, and alerts can be sent to facility staff to make adjustments promptly.
Such sensors are particularly vital for handling moisture-sensitive medications, such as capsules, powders, and certain types of injectable drugs. These medications can absorb moisture from the air, which can cause them to get spoiled faster.
8. Predictive quality testing
This is an innovative approach to quality control in the pharmaceutical industry that leverages IoT technology to conduct batch-wise quality testing. The end-to-end IoT solution uses a range of sensors and devices to collect data throughout the production and distribution process, providing real-time insights into the quality of drugs.
The process begins with collecting data from various sources, such as manufacturing equipment, transportation containers, and storage facilities. The system can generate alerts and notifications when potential quality issues are detected. This helps prevent batch recalls and other problems that can be costly and damaging to pharmaceutical companies.
Over to you
Maintaining all the parameters during pharmaceutical delivery and storage can be costly for all stakeholders. It may be more than they can handle. That is where deploying IoT technology for this task can be the best option for everyone involved.
IoT can lead to stable drug quality, improved shipment efficiency, business credibility, and customer service. If you want to know more about how IoT can be a game-changer for your business, schedule a free call with our experts at IntuThings.